Pup ultrasonic vocalizations triggering alloparental retrieval
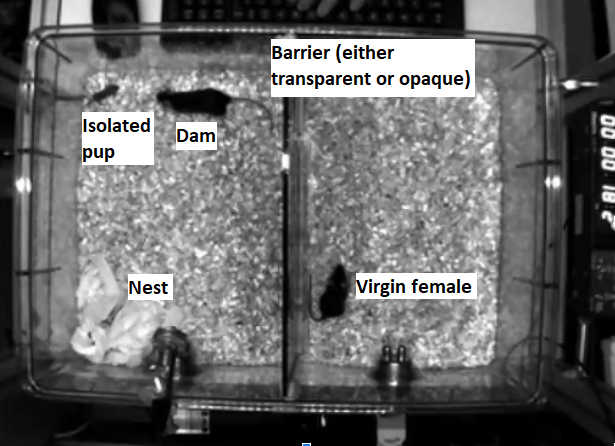
Figure 1: Observational PRT. Left, a dam runs towards her pup (upper left of the cage) to bring it back to the nest (lower left of the cage). Right, a pup-naive virgin female mouse observes through a transparent or opaque barrier.
Proper care for the offspring is essential for a species survival (Dulac et al, 2014). In rodents, a classic maternal behavior is retrieving pups that got astray back to the nest, and failure to perform this retrieval might otherwise cause pup hypothermia and consequent death (Hann and Lavooy, 2005).This behavior is stereotypical for dams (i.e., mother mice) but it doesn't appear to be innate for pup-naive, virgin females (Marlin et al 2015, Carcea et al 2021), which need longer exposure and contact with pups to be able to perform it and become alloparental. In the lab, this classic maternal behavior can be tested by the pup retrieval test (PRT), where individual pups are dislodged from the nest by an experimenter until they are retrieved back by an adult (either dam or virgin female) mouse (Hann and Lavooy, 2005).
Sensorimotor cues from pups (e.g, ultrasonic vocalizations (USV)) are thought to trigger the retrieval behavior (Schiavo et al, 2020, Okabe et al 2013), and are differentially processed by dams and virgin females (Lecca et al, 2022). However, the nature of this vocalization and whether specific features of it can trigger innate retrieval by nonparental/alloparental agents is not known. Given this scenario, correlating the features of the pup’s USVs (bouts, timing, duration, frequency, etc) with the performance of dams and alloparental females in the PRT could help elucidate how this behavior is initiated and triggered on alloparental agents.
In a recent project, we acquired a large dataset of observational PRT (i.e, a pup-naive, virgin female either observes a dam performing pup retrieval through a barrier and later is tested with the pups, Figure 1) together with recording of pups USVs, which remains to be extracted and analysed. Therefore, in this semester project proposal, it would be interesting to correlate the animal (both dams and/or virgin females and pups) position and behavior (e.g, by using Deep Lab Cut) with the pups USVs (e.g, extraction and analysis by using Deep Squeak).
REQUIREMENTS/PRE-REQUISITES
-
- Intermediate knowledge of programming (Python and/or MATLAB).
-
- Experience with DeepLabCut (recommended).
-
- Strong interest in neuroscience and animal behavior.
CONTACT
Eduarda Streit Morsch (eduarda@ini.ethz.ch)
PhD student at the Grewe Lab at the Institute of Neuroinformatics.
REFERENCES
-
- Dulac, Catherine, Lauren A. O’Connell, and Zheng Wu. "Neural control of maternal and paternal behaviors." Science 345.6198 (2014): 765-770.
-
- Hahn, Martin E., and Maria J. Lavooy. "A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents." Behavior genetics 35 (2005): 31-52.
-
- Marlin, Bianca J., et al. "Oxytocin enables maternal behaviour by balancing cortical inhibition." Nature 520.7548 (2015): 499-504.
-
- Carcea, Ioana, et al. "Oxytocin neurons enable social transmission of maternal behaviour." Nature 596.7873 (2021): 553-557.
-
- Schiavo, Jennifer K., et al. "Innate and plastic mechanisms for maternal behaviour in auditory cortex." Nature 587.7834 (2020): 426-431.
-
- Okabe, Shota, et al. "Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice." Behavioral neuroscience 127.3 (2013): 432.
-
- Lecca, Salvatore, et al. "A neural substrate for negative affect dictates parental behaviour." (2022).
-
- Mathis, Alexander, et al. "DeepLabCut: markerless pose estimation of user-defined body parts with deep learning." Nature neuroscience 21.9 (2018): 1281-1289.
-
- Coffey, Kevin R., Ruby E. Marx, and John F. Neumaier. "DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations." Neuropsychopharmacology 44.5 (2019): 859-868.