Firmware Light Sensing Applications in in-vivo Fiberphotometry
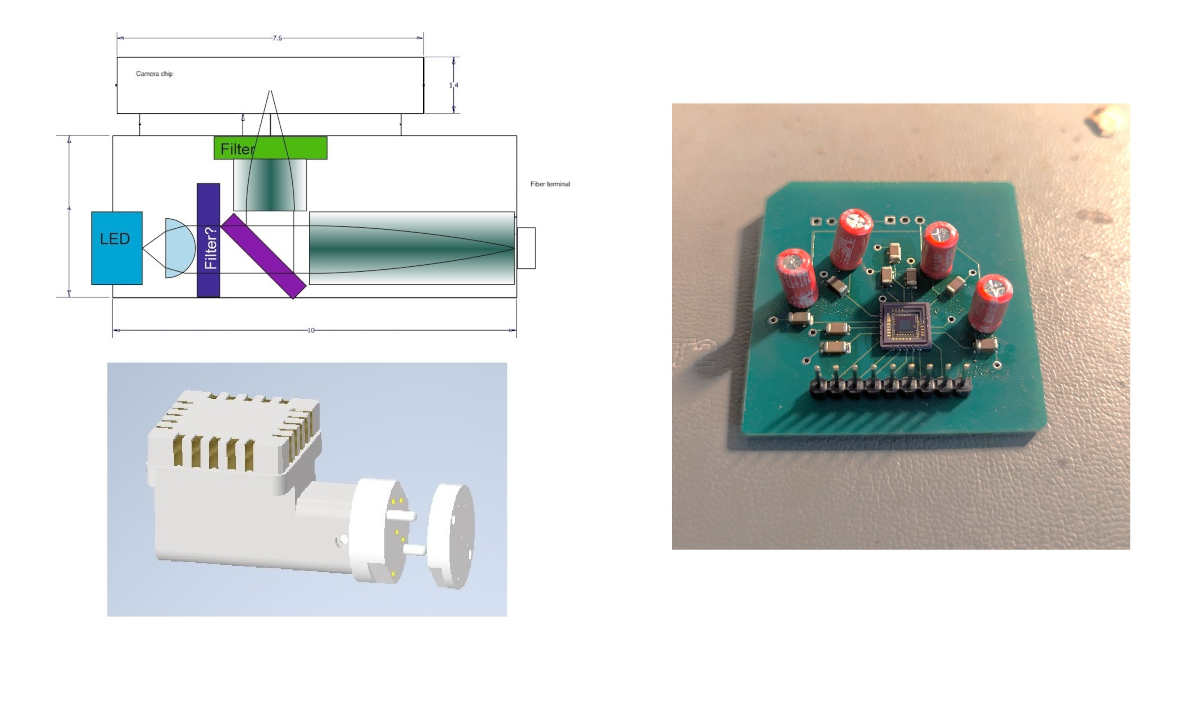
Figure 1: Headstage of fiber photometry device (left) and breakout (right) for the optical sensor (Hamamatsu S14250).
1 Overview and Background
Fiberphotometery is a powerful tool for studying the underlying mechanisms of neural circuits and linking them to behavior. An optic fiber is surgically implanted in a region of interest in a brain area that has been genetically modified to emit light. An excitation pulse is delivered through the optic fiber, and the emitted light response is measured using a photosensor [1] [2]. The current standard for fiberphotomtry studies requires birds to be tethered to a large mechanism that limits the birds’ movement [3]. Apart from concerns about animal welfare, this setup does not allow the animals to be studied under natural conditions including unconstrained social interactions between individuals.
Wireless systems have been designed to overcome these limitation. Examples are miniature microscopes for calcium imaging that have been tested both in mice and songbirds [4] [5]. These methods however limit photometry to a brain area that fits into the field of view, and consume large amounts of electrical power.
Similarly, multiple setups for wireless fiberphotometry have been developed and successfully tested in mice [6][7][8]. These however are based on a single photodiode yieling a mere scalar signal. We want do extend these developments and design a system suitable for multi-fiber photometry where fibers can be guided into regions of interest even if they are physically far apart [9]. Our goal is to create a setup which will allow us to perform fiber photometry from multiple fibers in a setup that does not require physical wires which constrains the movement of the animal.
2 Project
A core component of the mutli-fiber photometry setup is the optic sensor which records the light signal coming out of the excited tissue. This device needs to be placed on the bird’s head and is connected to a microntroller and from there to a low-power Bluetooth module placed on the bird’s back to transmit the digital signal to a host computer for analysis. Our setup is based on the low-energy light sensor Hamamtsu S14250. We created a breakout for this device (Fig 1 right) in order to be able to measure the device’s power consumption, its light sensitivity, and its latency.
This semester project will involve the following tasks:
- - Creating firmware for the MCU to initiate the sensor, receive data, transmit it over Bluetooth, and set the device to sleep mode
- - Measuring the power consumption of the device.
- - Quantify the signal-to-noise ratio in a mock setting
On the basis of these measurements, the student shall assess the feasibility of utilizing the device in a live setting, and make suggestions for the implementation.
3 Contact
For more details about this project please contact Etay Yacov from Hahnloser Lab: eyacov@ethz.ch
References
[1] Yuanmo Wang et al. “A selected review of recent advances in the study of neuronal circuits using fiber photometry”. In: Pharmacology Biochemistry and Behavior 201 (2021), p. 173113. issn: 0091-3057. doi: https://doi.org/10.1016/j.pbb.2021.173113. url: https://www.sciencedirect.com/science/ article/pii/S0091305721000113.
[2] Eleanor H. Simpson et al. “Lights, fiber, action! A primer on in vivo fiber photometry”. In: Neuron 112.5 (2024), pp. 718–739. issn: 0896-6273. doi: https://doi.org/10.1016/j.neuron.2023.11.016. url: https://www.sciencedirect.com/science/article/pii/S0896627323008905.
[3] Andrea Roeser et al. “Dopaminergic error signals retune to social feedback during courtship”. In: Nature 623.7986 (Sept. 2023), pp. 375–380. issn: 1476-4687. doi: 10.1038/s41586-023-06580-w. url: http: //dx.doi.org/10.1038/s41586-023-06580-w.
[4] William A Liberti III et al. “An open source, wireless capable miniature microscope system”. In: J. Neural Eng. 14.4 (Aug. 2017), p. 045001.
[5] Feng Xue et al. “Multi-region calcium imaging in freely behaving mice with ultra-compact head-mounted fluorescence microscopes”. In: National Science Review 11.1 (Nov. 2023), nwad294. issn: 2095-5138. doi: 10.1093/nsr/nwad294. eprint: https://academic.oup.com/nsr/article-pdf/11/1/nwad294/ 57318999/nwad294.pdf. url: https://doi.org/10.1093/nsr/nwad294.
[6] Etienne Ransford et al. “Electro-Optic Fiber Photometry Front-End for Live Wireless Brain Calcium Sensing Applications”. In: IEEE Sensors Journal 23.24 (2023), pp. 30302–30310. doi: 10.1109/JSEN. 2023.3326740.
[7] Mehdi Noormohammadi Khiarak et al. “A Wireless Fiber Photometry System Based on a High Precision CMOS Biosensor With Embedded Continuous-Time Σ∆ Modulation”. In: IEEE Transactions on Biomedical Circuits and Systems 12.3 (2018), pp. 495–509. doi: 10.1109/TBCAS.2018.2817200.
[8] Luyao Lu et al. “Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain”. In: Proceedings of the National Academy of Sciences 115.7 (2018), E1374–E1383. doi: 10.1073/ pnas . 1718721115. eprint: https : / / www . pnas . org / doi / pdf / 10 . 1073 / pnas . 1718721115. url: https://www.pnas.org/doi/abs/10.1073/pnas.1718721115.
[9] Yaroslav Sych et al. “High-density multi-fiber photometry for studying large-scale brain circuit dynam ics”. In: Nature Methods 16.6 (June 2019), pp. 553–560. issn: 1548-7105. doi: 10.1038/s41592-019- 0400-4. url: https://doi.org/10.1038/s41592-019-0400-4.